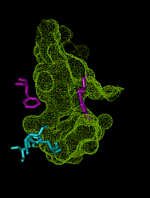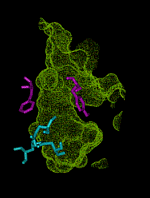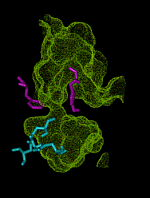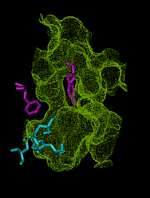

This animation shows the ``breathing'' motions of the gorge or channel that leads from the region outside the enzyme acetylcholinesterase (AChE), to the active site. These fluctuations in the width of the channel are required to allow the substrate acetylcholine (ACh) to move from the outside into the active site. They also contribute to the selectivity of the enzyme, by slowing the entrance of substrates that are larger than ACh. This animation is composed of 75 frames set up to loop for 1 000 iterations.
The outer entrance to the channel is at the top in this animation, and the active site chamber, with key amino acid residues shown in blue, is at the bottom. The structures are from a 750 ps molecular dynamics simulation (Wlodek et al., 1997). The surface of the channel defines the volume that is accessible to a probe of radius 0.14 nm, about the size of a water molecule. The bottleneck region midway down the channel is formed by aromatic residues including Phe 290 and Tyr 334, shown in purple. This bottleneck is almost always closed to the substrate ACh, which can be approximated by a probe of radius 0.24 nm. Nevertheless, the fluctuations in the width of the bottleneck allow ACh to successfully enter the active site almost every time an ACh molecule moves into the outer part of the channel. Substrates that are even a little bit larger than ACh are much less likely to be admitted before they escape back to the outside, because the bottleneck opens less frequently for larger substrates. Thus, the enzyme exhibits ``dynamic selectivity'' in its binding of substrates (Zhou et al., 1998).
Joel Sussman kindly provided the crystallographic coordinates that were used to start the simulation (Harel et al., 1993), and are available from the Protein Data Bank (PDB) code: 1ACJ). Jim Briggs and Paul de Bakker helped in the preparation of this animation. This work was supported in part by grants from NSF, NIH, and the San Diego Supercomputer Center.